FDA approves over-the-counter sensor for glucose monitoring
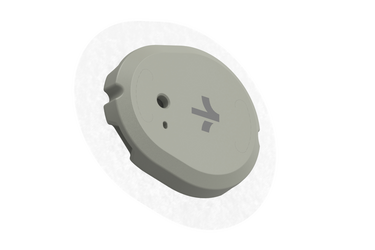
The world's first over-the-counter glucometer from Dexcom will be officially available for sale this summer. The manufacturer announced that a new continuous glucose monitor called Stelo has been approved by the FDA for use. A small sensor is attached to the shoulder, which allows you to monitor the level of glucose in the blood 24/7.
The device is intended for patients with type 2 diabetes who do not use insulin. Dexcom intends to sell glucometers at the most affordable price possible and to engage insurance companies for partial or full insurance coverage.
What is a continuous glucose monitor?
According to the International Diabetes Federation IDF, about 537 million people are living with diabetes today, 95% of whom have type 2 diabetes. One of the main conditions for the prevention, control, and successful treatment of the disease is the control of the level of glucose in the blood.
Continuous glucose monitors or CGMs from the American manufacturer Dexcom are small sensors that allow real-time monitoring of glucose levels through the skin. They are mostly used by patients with diabetes. Information is transmitted wirelessly to a smartphone, alerting users, their families, and doctors to emergency situations.
Dexcom's new CGM is designed for patients with type 2 diabetes who do not use insulin. This is the first non-prescription glucose biosensor. According to the manufacturer, this means Stelo will be available to people without CGM insurance.
Stelo will become more available in the US and globally
According to Dexcom, more than 25 million patients with type 2 diabetes in the US do not use insulin. Although the G7 CGM system is available for this population, patients must obtain a prescription. As a result, the device is not available to people without insurance.
"CGMs can be a powerful tool for monitoring blood glucose levels. The current approval expands access to these devices by allowing people to purchase CGMs without the involvement of a healthcare provider," said director of the FDA's Center for Devices and Radiological Health, Dr. Jeff Shuren.
The device, called Stela, was submitted to the FDA for review in February of this year. According to Dexcom, the sensor should be worn on the shoulder for 15 days, after which it should be replaced.
The sensor has been simplified for easier use
According to Dexcom COO Jake Leach, Stelo will have a unique platform and branding. It will be adapted to the needs of patients with type 2 diabetes. This means that the platform will not include many of the alerts and notifications intended for diabetes patients who are at risk of a more serious emergency.
"The device is designed to be easier to use. There are a lot of people who could benefit from it," said Leach.
According to the expert's predictions, insurance companies should be interested in the advantages of Stelo, fully covering the insurance. Dexcom decided to first bring the product to market at an affordable price to get it into the hands of users quickly, Leach added.
"I think people need to have access to such a device, it's like a mirror of their body. It's too personal," the expert is confident.
Dexcom's continuous glucose monitor is expected to be available in summer 2024. The over-the-counter device will be marketed at an affordable price to make it as affordable as possible for millions of people with diabetes. Company representatives also hope that insurance agents will fully cover the cost of the device.
